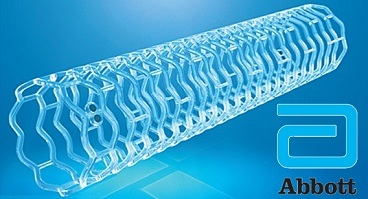
Abbott announced that the Ministry of Health, Labor and Welfare in Japan has approved the company's Absorb bioresorbable heart stent, making the first-of-its-kind medical device commercially available to treat people in Japan with coronary artery disease.
Absorb is the only fully dissolving heart stent approved in Japan for the treatment of heart disease, one of the leading causes of death in Japan and worldwide. An estimated 1.73 million2 people in Japan (population: 127 million) have heart disease, and approximately 71.7 thousand3 people die each year from coronary artery disease, making it the secondary leading cause of death in the country annually. Worldwide, more than 7.4 million people die of heart disease each year.4
"Absorb has the potential to address the unsolved challenges of managing coronary artery disease with conventional drug eluting metallic stents," said Prof. Takeshi Kimura, M.D., Ph.D., director of cardiovascular medicine at Kyoto University Hospital in Japan and the principal investigator of ABSORB JAPAN, the clinical study that led to the MHLW's approval of the device. "Our research, which involved 400 patients at 38 Japanese sites, shows that this fully dissolving stent delivered comparable results to the best-in-class Abbott XIENCE® metallic drug-eluting stent on clinically measures of safety and efficacy."
Japan ranks as the second-largest single-country market for advanced medical technology behind the United States, where the U.S. Food and Drug Administration (FDA) approved Absorb in July 2016.
While stents are traditionally made of metal, Abbott's Absorb stent is made of a naturally dissolving material, similar to dissolving sutures. Absorb disappears completely5 in approximately three years, once it has done its job of keeping a clogged artery open and prmopting healing of the treated artery segment. By contrast, metal stents are permanent implants that may cause future blockages.
"We're very excited to bring the promise of Absorb to patients in Japan," said Deepak Nath, Ph.D., senior vice president, vascular, Abbott. "We believe the Absorb bioresorbable stent can help people live better, healthier lives without the concern of a having a metal implant."
Abbott's Absorb stent, sold commercially as the Absorb GT1 Bioresorbable Vascular Scaffold (BVS) system, has been used to treat more than 150,0001 people in more than 100 countries around the world.Absorb was recently approved in the United States and Canada.
ABOUT ABBOTT:
At Abbott, we're committed to helping you live your best possible life through the power of health. For more than 125 years, we've brought new products and technologies to the world — in nutrition, diagnostics, medical devices and branded generic pharmaceuticals that create more possibilities for more people at all stages of life. Today, 74,000 of us are working to help people live not just longer, but better, in the more than 150 countries we serve.
Connect with us at www.abbott.com
For further information: Media:
Kristina Becker,
Abbott +1 408-845-3427,
or
Toshiyuki Shimizu,
Abbott Japan,
+81 3-4555-1002,
or
Financial:
Mike Comilla,
Abbott +1 (224) 668-1872